In nature, some of the most unexpected sources hide remarkable “biotechnologies” – such as spider venom. Once regarded as "lethal weapons," these neurotoxins are now emerging as molecular tools for the precise modulation of neuronal signals, assisting the development of innovative drugs for treating neurological disorders such as epilepsy and chronic pain.
On July 4, 2025, the team led by Professor Nieng Yan and Dr Jian Huang at the Shenzhen Medical Academy of Research and Translation (SMART) published an online research paper in Cell Research titled “Phrixotoxin-3 binds to three distinct antagonistic sites on human Nav1.6”. This study, for the first time, revealed that the spider toxin Phrixotoxin-3 (PaurTx3) modulates the human voltage-gated sodium channel 1.6 (Nav1.6) through a unique three-site binding mechanism, providing novel insights for peptide-based drug design.

Nav channels serve as essential “switches" in the nervous system, rapidly responding to changes in membrane potential to regulate the initiation and conduction of nerve impulses. Nav1.6 subtypes are widely distributed in the central nervous system and act as the "primary channel" for neuronal excitability. Therefore, Nav1.6 dysfunction is closely linked to neurological disorders such as epilepsy. Despite its critical role, there are very few drugs that specifically target Nav1.6, making the development of selective modulators a major change in neuropharmacology.
PaurTx3, a neurotoxin derived from tarantulas, naturally paralyzes the nervous system of prey, assisting their capture. The research group, for the first time, resolved the complete structure of human Nav1.6 bound to PaurTx3 using cryo-electron microscopy. Surprisingly, PaurTx3 precisely binds to Nav1.6 with three distinct antagonistic sites, rather than acting through a single site. This new finding reveals a novel “three-lock” binding mode between Nav channels and neurotoxins, providing new ideas for the design of more specific and effective drugs for neurological disorder therapies.
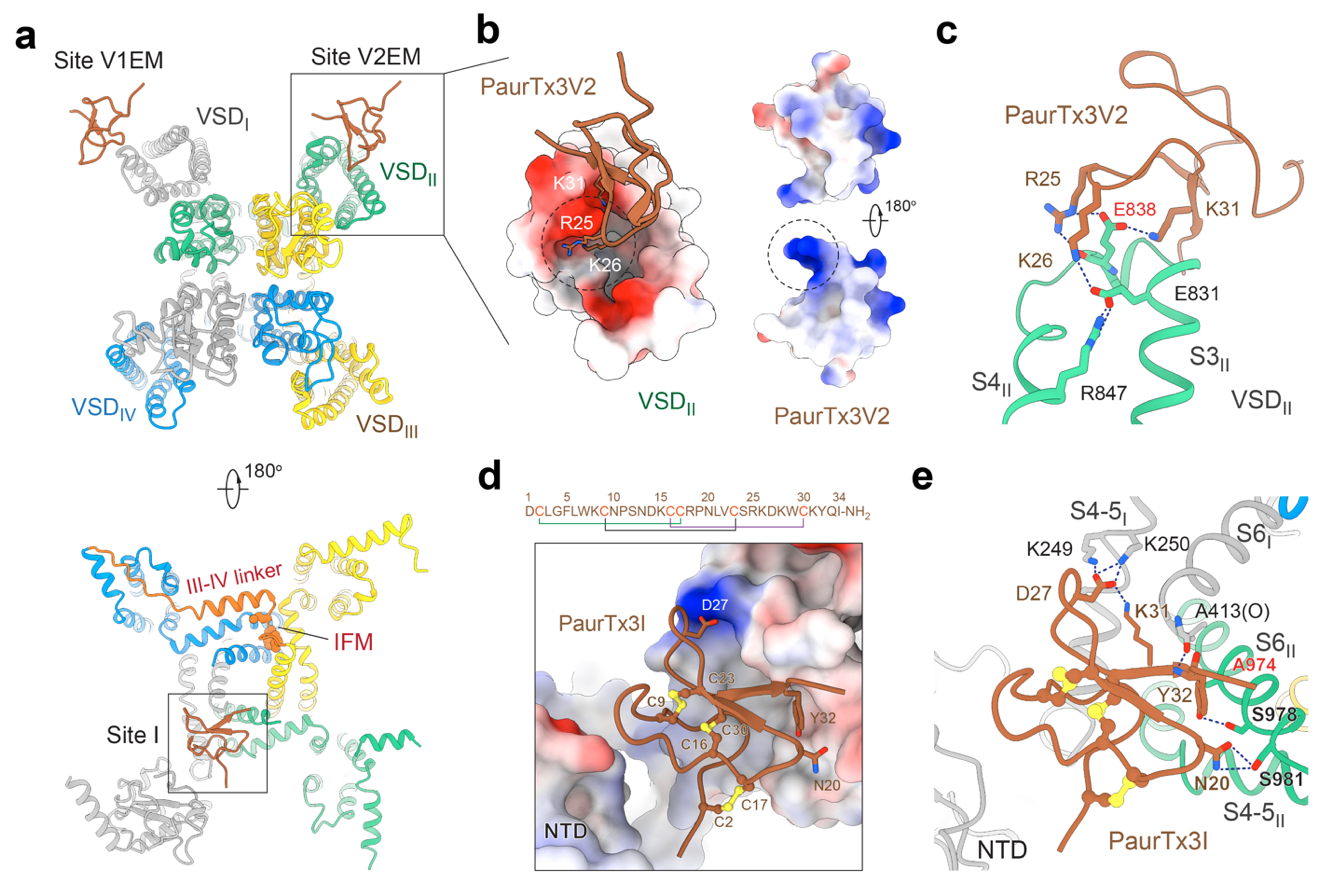
Fig. Spider toxin Phrixotoxin-3 coordinately modulates the Nav1.6 channel through three binding sites
Lock 1 & 2: Precise Binding to Voltage-Sensing Domains I and II (VSDI and VSDII).
The voltage-sensing domains (VSDs) of Nav channels are key components for sensing electrical signaling. PaurTx3 employs positively-charged residues to form electrostatic interactions with unique negatively-charged pockets on the VSDs, thereby "locking" the channel in an inactivated state. Mutation of the critical residue E838 significantly reduces toxin binding, indicating the importance of charge distribution for the channel-toxin interaction.
Lock 3: Groundbreaking Target to an Intracellular Gating Site
PaurTx3 also binds an intracellular gating site (site I), interfering with intracellular Na+ flux. Although the exact transmembrane entry route requires further validation, this "intramembrane assault" overcomes the limitation of drugs acting only on extracellular and intracellular mechanisms, and offers a new perspective for designing next-generation drugs with "synergistic external-internal" mechanisms.
Beyond elucidating the multi-site regulatory mechanism of Nav1.6, this study also provides valuable inspiration for structure-guided peptide drug design:
Optimizing charge matching to enhance drug binding affinity by optimizing electrostatic complementarity between drug and target protein.
Engineering transmembrane modulation to design drug fragments with membrane-penetrating capabilities that directly function at critical intracellular regulatory sites, expanding the targetable space.
Integrating multi-target strategies to develop peptide drugs that simultaneously act on multiple sites to improve therapeutic efficacy and specificity.
Furthermore, peptide toxins serve as precision-targeting molecular tools, providing a paradigm for the structural optimization, mechanism validation, and preclinical development.
Scientists are transforming "natural weapons" into "medical tools," from natural toxins to precision medicines. The Yan/Huang group is dedicated to working on analyzing Nav1.6 structures and exploring peptide toxin mechanisms, holding promise for developing anti-epileptic, analgesic, and neuroprotective drugs targeting Nav1.6 with novel functional modes.
Corresponding Authors:
Ning Yan, Investigator at the Beijing Frontier Research Center for Biological Structure, Founding President of SMART, and Director of Shenzhen Bay Laboratory (SZBL)
Jian Huang, Principal Investigator at SMART
Co-First Authors:
Xiao Fan, Former Postdoctoral Fellow at Department of Molecular Biology at Princeton University
Jiaofeng Chen, Postdoctoral Fellow at School of Life Sciences at Tsinghua University
Other Authors:
Xiaoshuang Huang, Associate Researcher at SMART
Yuzhen Xie, Research Assistant at SMART
Zhanfeng Hou, Ph.D. student at Peking University Shenzhen Graduate School (PKUSZ),
Zigang Li, Senior Investigator at Pingshan Biopharmaceutical R&D and Translation Center in SZBL and PKUSZ
Fundings:
Supported by the National Natural Science Foundation of China, the Human Frontier Science Program (HFSP) Long-Term Fellowship, and the start-up fund from SMART.