Voltage-gated ion channels (VGICs) represent a large family of membrane proteins, including voltage-gated sodium (Nav), calcium (Cav), and potassium (Kv) channels. These channels sense changes in transmembrane potential and selectively regulate the transport of specific ions across the cell membrane. As key mediators of bioelectrical signaling, they play essential roles in neurotransmission, muscle contraction, and hormone secretion. Dysfunctions in VGICs, whether due to aberrant channel activity or impaired regulation, are associated with a broad range of diseases, including epilepsy, neuropathic pain, cardiac arrhythmias, and even sudden death. Consequently, VGICs are considered as important drug targets. Indeed, approximately 10% of all FDA-approved drugs act directly on VGICs. Therefore, a comprehensive understanding of the structure–function relationships of VGICs is critical for elucidating their mechanisms of action and for advancing rational drug discovery.
In 1791, Luigi Galvani demonstrated that electrical stimulation of a dead frog’s leg could induce muscle contraction, and since then, bioelectric phenomena have remained a central focus of physiology. Over the past two centuries, major contributions have been made by scientists, including Alan L. Hodgkin and Andrew F. Huxley, who identified distinct ionic currents, characterized the action potential and resting potential, and established the foundations of electrophysiology. Advances such as the development of patch-clamp recording enabled the measurement of currents through individual ion channels, while molecular cloning allowed the identification of VGICs and characterization of their biophysical properties. However, structural studies lagged behind; apart from the determination of a potassium channel structure in 1998, sodium and calcium channel structures remained elusive for many years, thereby limiting progress in mechanism-based drug development.
In metazoans, electrical signals, most prominently the action potential, are indispensable for nearly all physiological and neural processes. Action potentials can propagate along myelinated axons at speeds up to 120 m/s, enabling rapid responses to stimuli. The initiation of the action potential relies on Nav and Cav channels, its propagation is mediated by Nav channels, and its termination depends on Kv channels. In other words, the opening and closing of these channels are precisely regulated by changes in membrane potential. Even subtle perturbations in this process can profoundly affect neuronal and muscular excitability, leading to pathophysiological conditions. Accordingly, numerous therapeutic agents—including antiepileptic, antiarrhythmic, antihypertensive, and antidepressant drugs, as well as anesthetics—achieve their effects by modulating VGICs.
In recent years, the resolution revolution of single-particle cryo-electron microscopy (cryo-EM) has provided unprecedented opportunities for structural studies of VGICs, yielding high-resolution structures of multiple representative channels. These advances have greatly enhanced our mechanistic understanding of VGICs, including their voltage-sensing mechanisms (how channels detect changes in transmembrane potential), electromechanical coupling (how voltage sensing drives pore opening), ion selectivity (how channels discriminate among ions), and inactivation (how channels terminate signaling to prevent sustained neuronal firing). Moreover, the determination of channel–ligand complex structures has illuminated the mechanisms by which drugs and toxins modulate channel activity, identified novel pharmacological binding sites, and provided valuable insights for structure-based drug design.
The Nieng Yan’s group has long been devoted to the study of Nav and Cav channels, maintaining an internationally leading position in structural and pharmacological research on VGICs. On August 5, 2024, Professor Nieng Yan, Dr. Xiaojing Pan, and Dr. Jian Huang published a review article entitled “Structural Biology and Molecular Pharmacology of Voltage-Gated Ion Channels” in Nature Reviews Molecular Cell Biology. This review summarizes the most recent progress in structural studies of VGICs, provides a detailed discussion of their functional mechanisms, and highlights how drugs and toxins regulate VGIC activity, thereby laying the foundation for rational, structure-based drug discovery.
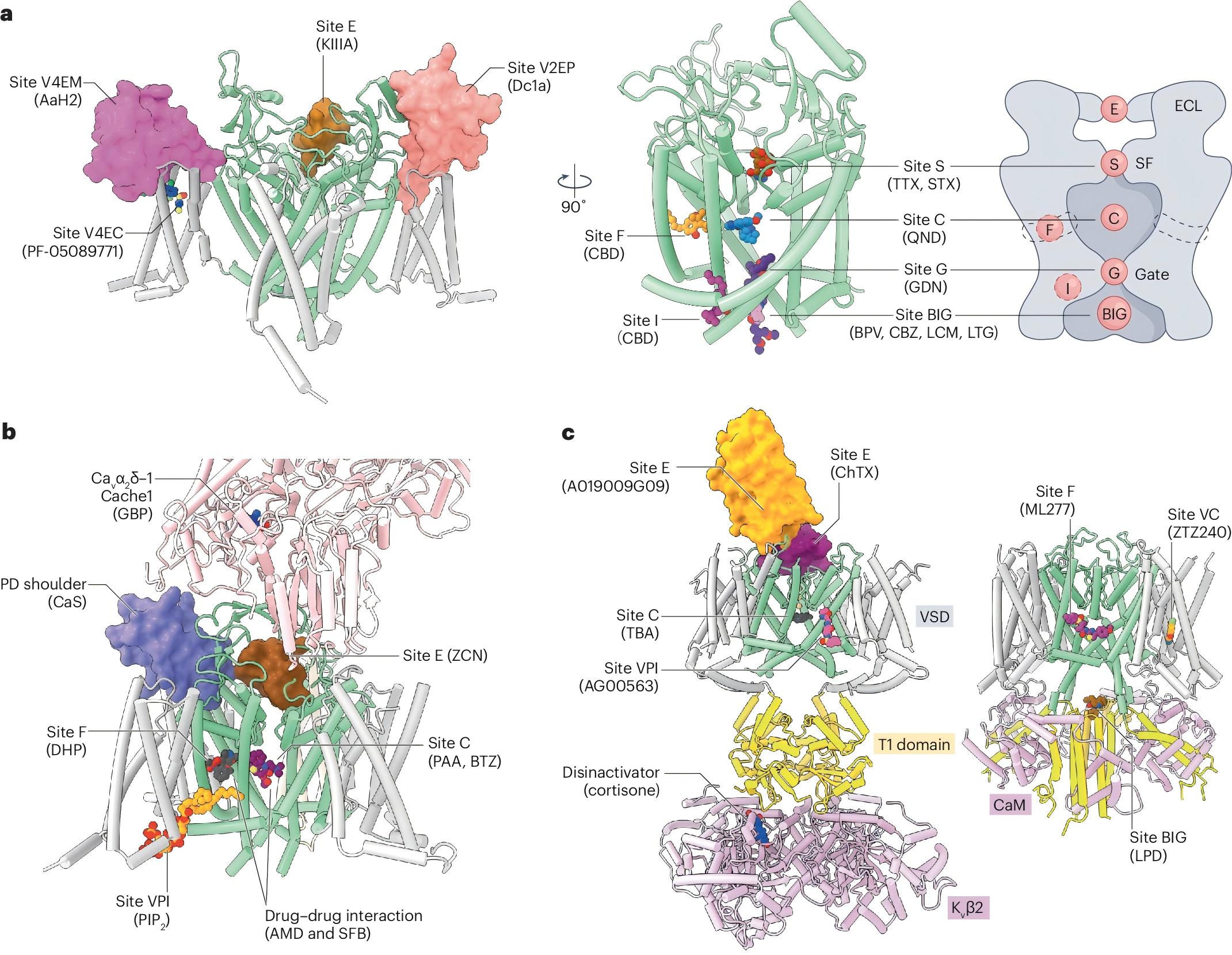
The review also discusses recent advances in structural biology, particularly the application of artificial intelligence–assisted protein structure prediction. While AI-based approaches have produced remarkable results, challenges remain in accurately predicting the binding sites of small-molecule or peptide modulators, largely due to the limited number of experimentally resolved channel–ligand complexes. Nevertheless, with the continuous expansion of structural databases and the rapid evolution of AI technologies, significant breakthroughs in ligand binding and protein dynamics prediction can be anticipated in the near future.
This work was co-corresponded by Professor Nieng Yan, Chair Professor at Tsinghua University, Principal Investigator at the Beijing Advanced Innovation Center for Structural Biology, and Founding President of the Shenzhen Medical Academy of Research and Translation, together with Dr. Xiaojing Pan, Research Fellow at the Shenzhen Medical Academy of Research and Translation. The first author is Dr. Jian Huang, a postdoctoral researcher in the Department of Molecular Biology at Princeton University, who will soon join the Shenzhen Medical Academy of Research and Translation as a Research Fellow. The study was supported by the National Natural Science Foundation of China. Additional contributions were made by Chen Song (Peking University), Zhangqiang Li (Tsinghua University), Guanghui Yang (China Agricultural University), Huaizong Shen (Westlake University), Shuai Gao (Wuhan University), and Xia Yao (Wuhan University).