Pain, one of humanity's most fundamental sensations, remains a persistent medical challenge. From the anesthetic effects of coca leaves to modern precision therapeutics, scientists have continuously sought to uncover its mechanisms. Recently, Journavx (also known as VX-548, or Suzetrigine), a novel non-opioid analgesic drug that approved by FDA for targeting sodium channels in sensory neurons, has begun to be used in the treatment of chronic pain. This breakthrough provides safer and more effective therapeutic options for nearly one-third of the global population affected by chronic pain, particularly the elders.
On April 28, 2025, the research group led by Professor Nieng Yan and Dr Jian Huang at the Shenzhen Medical Academy of Research and Translation (SMART) published a review article in the Journal of General Physiology titled “Sensory Neuron Sodium Channels as Pain Targets: From Cocaine to Journavx (VX-548, suzetrigine)”. This article systematically reviewed over a century of scientific progress in targeting sensory neuron sodium channels for pain intervention.

In the late 19th century, scientists discovered the local anesthetic properties of cocaine and unveiled the therapeutic potential of sodium channel blockers. This finding led to the development of local anesthetics such as procaine and lidocaine, which became cornerstones of surgical anesthesia and local pain management. However, due to their broad activity and lack of selectivity, these traditional agents often cause side effects such as numbness and arrhythmia, limiting their use in chronic pain treatment.
By the late 20th century, advances in molecular biology enabled the successful cloning of multiple voltage-gated sodium channel (VGSC) subtypes, particularly Nav1.7, Nav1.8, and Nav1.9. They have emerged as promising analgesic targets because of their selective expression in sensory neurons. For instance, Nav1.7 has attracted significant attention due to its close association with hereditary pain disorders. Loss-of-function mutations in Nav1.7 cause congenital insensitivity to pain with anhidrosis, while gain-of-function mutations lead to severe pain disorder. However, its broad expression on the autonomic nervous system raises wide off-target effects, which narrow its therapeutic window.
In contrast, Nav1.8 is selectively expressed in peripheral sensory neurons, where it plays a central role in inflammatory and mechanical pain. Inhibitors of Nav1.8 have been shown to significantly reduce pain with minimal side effects, positioning it as a promising therapeutic target. Research on Nav1.9 remains limited, and its selective targeting strategies are still in nascent stages.
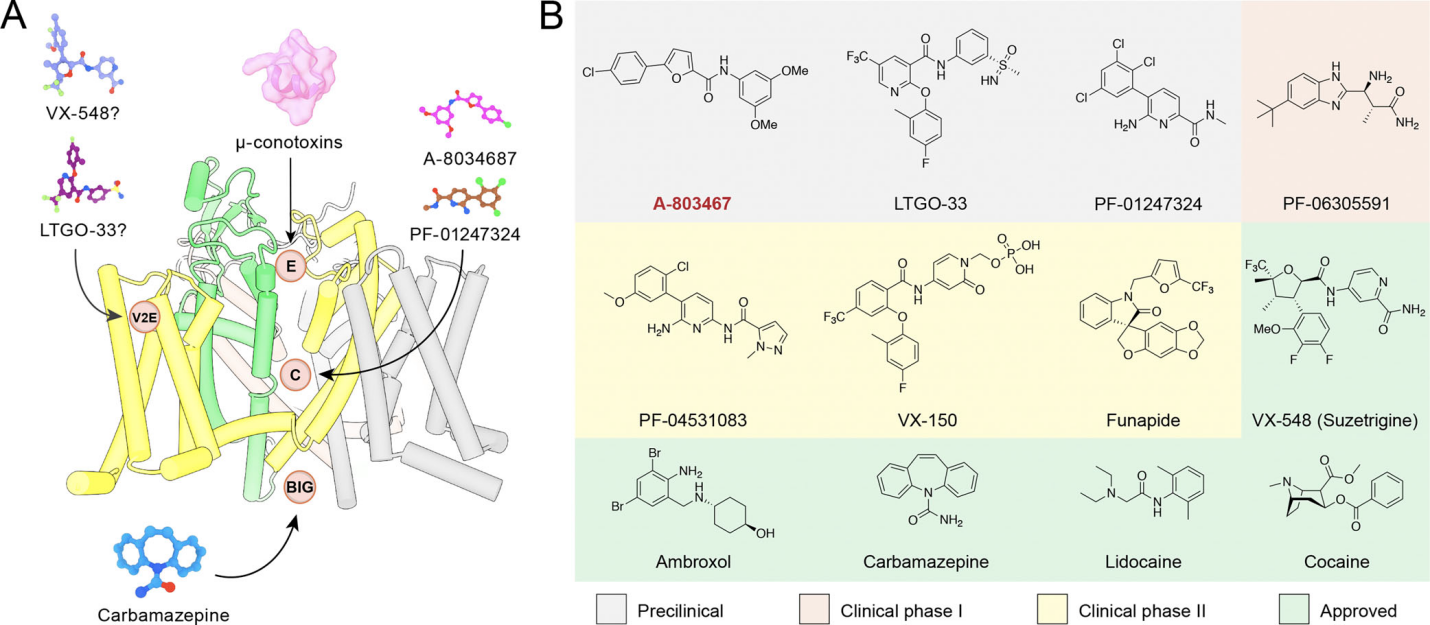
Fig. Summary of clinical or investigational NaV1.8 antagonists.
Journavx (VX-548/Suzetrigine), a Nav1.8-specific targeting antagonist developed by Vertex Pharmaceuticals, was recently approved by FDA, making the advent of a new era for sodium channel-targeted analgesics. Studies have demonstrated its selective binding features to the second voltage-sensing domain (VSD-II) of Nav1.8, allosterically stabilizing its closed state to block pain signal transmission. Journavx not only exhibits high selectivity and excellent oral bioavailability but also provides sustained analgesia with a favorable safety profile, achieving significant success in clinical trials.
Concurrently, next-generation sodium channel modulators, such as LTG-01 developed by Latigo Company, are under active development. Further elucidation of drug-channel interaction mechanisms using structural biology approaches, such as cryo-electron microscopy (cryo-EM), holds promise for driving the design of more precise and effective second-generation analgesics.
From the serendipitous discovery of cocaine’s local anesthetic properties to the rational drug design and approval of Journavx, sodium channel research has reached many milestones through the last decades. This progress not only ushers in a new era of pain treatment but also vividly demonstrates how fundamental research drives clinical translation and improves patients’ lives.